 When two different metals come in contact with each other in presence of an electrolyte (e.g. water), they form a galvanic cell in which the lesser noble metal (e.g. Zn) corrodes in favour of the more noble metal (e.g. steel). This electrochemical reaction is the base for the complex field that is cathodic protection.
When two different metals come in contact with each other in presence of an electrolyte (e.g. water), they form a galvanic cell in which the lesser noble metal (e.g. Zn) corrodes in favour of the more noble metal (e.g. steel). This electrochemical reaction is the base for the complex field that is cathodic protection.
Galvanic, cathodic protection, or active protection, arises from zinc (the anode) sacrificing itself in favour of the base metal -steel (the cathode) with the resulting flow of electrons preventing steel corrosion. In this way the protection of the metal is guaranteed, even when the zinc layer is slightly damaged.
Other well-established methods of cathodic protection include hot-dip galvanising (HDG) and zinc thermal spraying, both of which exhibit a constant sacrificial rate of the zinc layer.
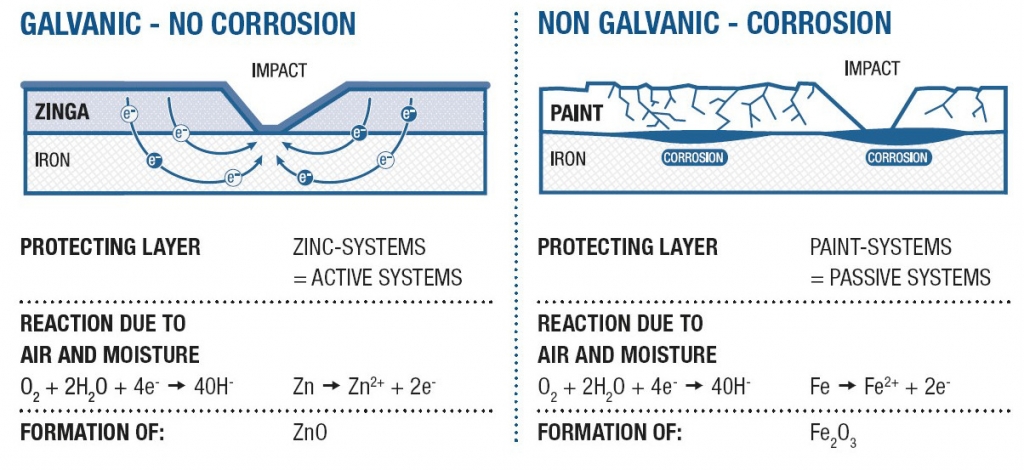
 With ZINGA the sacrificial rate reduces dramatically after the zinc layer has oxidised and the natural porosity has been filled with zinc salts. Additionally the zinc particles within the ZINGA layer are protected by the organic binder without adversely affecting the electrical conductivity. This enables ZINGA to create nearly the same galvanic potential between the zinc and the steel as hot dip galvanising but with a lower rate of zinc loss because, put simply, the binder acts as a “corrosion inhibitor” to the zinc.
With ZINGA the sacrificial rate reduces dramatically after the zinc layer has oxidised and the natural porosity has been filled with zinc salts. Additionally the zinc particles within the ZINGA layer are protected by the organic binder without adversely affecting the electrical conductivity. This enables ZINGA to create nearly the same galvanic potential between the zinc and the steel as hot dip galvanising but with a lower rate of zinc loss because, put simply, the binder acts as a “corrosion inhibitor” to the zinc.
Referenced from: www.zinga.eu/how-does-it-work
